| Posted: Sep 29, 2008 | |
Shaken, not stirred - how sonication can change the electronic structure of carbon nanotubes |
|
| (Nanowerk Spotlight) In chemistry, the process of doping refers to adding impurities to a very pure - or intrinsic - substance in order to alter its properties. Doping is used in the semiconductor industry to control the electrical resistivity of a material. Doping is also used by nanotechnology researchers to alter the electronic properties of carbon nanotubes, especially single-walled carbon nanotubes (SWCNT) that are intensely investigated for use in nanoelectronics. | |
| Since SWCNTs are sensitive to their chemical environment, they can be intentionally doped by a variety of dopants such as iron chloride, ammonia or nitrogen dioxide. Depending on what kind of elements are added, researchers speak about n-doping (n stands for negative) and p-doping (p stands for positive), where the electrical conductivity of SWCNTs is modified by either electron donors or electron acceptors. For instance, ammonia adsorbed on nanotube walls leads to n-doping while oxygen and solid organic acids lead to p-doping. | |
| So far, so good. However, scientists have known for some time that something funny happens when nanotubes are sonicated in certain solvents – sometimes their electronic properties change. Since mass produced nanotubes are clumped into bundles and ropes, they need to be dispersed prior to further processing in order to separate the individual nanotubes. A popular way of doing this is by exposing the CNT samples to ultrasonic pressure waves (ultrasonication). Adding a dispersing reagent (surfactant) into the solution will accelerate the dispersion effect. | |
| "Nobody has ever bothered to track down what really happens to the nanotubes during sonication, how their electronic structure changes and why" Dr. Peter Kruse tells Nanowerk. "The reason nobody has bothered – as far as I can discern – is because the effect was difficult to reproduce since not all factors were understood and it was seen as peripheral and distracting from the far more sexy pursuit of 'making something useful' like nanocomposite, devices, etc." | |
| Kruse, an Associate Professor of Chemistry at McMaster University in Canada, together with his graduate student Kevin R. Moonoosawmy, has just published a paper in which they shed light on how and why the electronic structure of carbon nanotubes changes during sonication ("To Dope or Not To Dope: The Effect of Sonicating Single-Wall Carbon Nanotubes in Common Laboratory Solvents on Their Electronic Structure"). | |
|
|
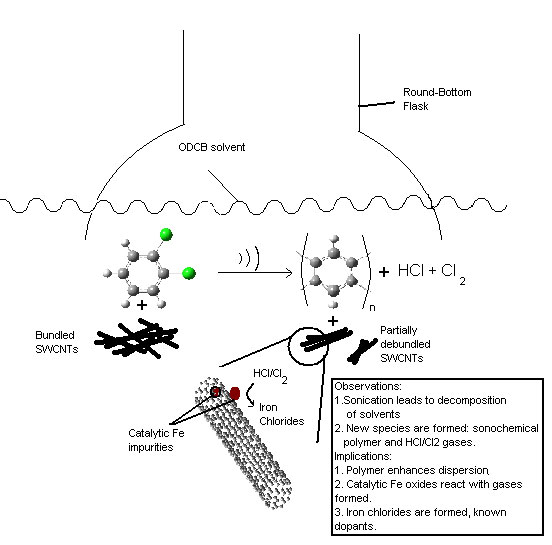
| Schematic representation of the effect of sonicating SWCNTs. (Image: Kevin R. Moonoosawmy) | |
| Kruse explains that at the outset of their experiments he and Moonoosawmy were not interested at all in the details of nanotube sonication. "We were interested in understanding the spatial distribution of functional groups along carbon nanotube sidewalls, based on the premise that the first reaction site would effectively act as a defect that would spatially modulate the electronic structure of the nanotubes and lead to differential reactivity during subsequent attachment. We learned the hard way that wet-chemical sample preparation was playing tricks on us and to get reproducible data we had to go back and understand every step along the way. That's how this paper came about as the first in a series which is currently in the works." | |
| The scientists noticed unexplained peak shifts in the Raman spectra of nanotubes that had been sonicated in chlorinated solvents. Raman spectroscopy can be used to follow the doping of nanotubes and the peaks shifts during processing indicated doping. | |
| "We clearly established that the electronic band structure of SWCNTs is disrupted by sonication in chlorinated solvents in the presence of iron nanoparticles" says Kruse. "Sonication not only is routinely used to disperse SWCNTs but also inevitably decomposes the solvent to form new species such as hydrogen chloride and chlorine gases. These, in turn, react with iron nanoparticles to form iron chlorides, a known dopant" (iron is often used as a catalyst for nanotube growth, but it is difficult to eliminate entirely from later processing and iron nanoparticles remain attached to the nanotubes). | |
| Kruse's study provides a platform on which future work, such as intentional doping – or avoidance of unintentional doping – of the SWCNTs using ionic liquids or salts for use in molecular electronics, can be achieved. In essence, though, it is a piece of fundamental science understanding of what wet-chemical processing does to nanotubes. You could argue that any scientists trying to do anything with nanotubes that involves wet-chemical processing should be interested in these results, since these findings will educate the way these processes are engineered. | |
| Kruse notes that, while this advance might appear marginal, is is important in understanding essential processes that occur during preparation of nanotubes for later processing. " We and other groups can build on these findings by either avoiding the solvents in question or intentionally using solvents to achieve desirable modifications of the nanotube properties when we design chemical reactions involving carbon nanotubes." | |
| According to Kruse, progress in developing functional carbon nanotube applications has been slower than originally promised because carbon nanotube research and engineering has been plagued by a lack of understanding of the fundamental processes involving nanotubes. | |
| "Our goal is to change that" he says. "Systematically digging through the fundamental science of carbon nanotube processing is more tedious and less glorious, but ultimately necessary in order to make good on the promises to develop fancy gadgets based on carbon nanotubes." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
