| Posted: Jun 22, 2009 | |
Nanotechnology approach for advanced drug delivery of water-insoluble therapeutics |
|
| (Nanowerk Spotlight) A broad spectrum of therapeutics or effector molecules that address several areas of medicine, from inflammation, to cancer, and regenerative medicine, are insoluble in water (they are soluble primarily in solvents generally regarded as unsuitable for injection). The water insolubility of these therapeutics limits the means by which those compounds can be administered to the body. Rapid strategies to package and disperse these drugs in biocompatible vehicles while also maintaining their potent activity can have major implications in advancing fundamental, translational, and commercial/scale-up aspects of accelerating their clinical impact. A new study now shows a way in which nanodiamonds can be applied towards enhancing water dispersion of otherwise poorly watersoluble therapeutics. It realizes a high throughout strategy to solubilize a broad range of water-insoluble drugs, which coupled with the innate biocompatibility of nanodiamonds, provides an important foundation towards a nanotechnology platform approach for advanced drug delivery. | |
| "Our work has realized the application of nanodiamond particles as vehicles for delivering water-insoluble therapeutics for applications in breast cancer (4-Hydroxytamoxifen) and liver cancer therapy (Purvalanol A)," Dean Ho tells Nanowerk. "We have shown through multiple modes of characterization, including UV-vis spectrophotometry, transmission electron microscopy (TEM) imagery, and zeta potential measurement/dynamic light scattering analysis that the water-insoluble therapeutics physically interact with the nanodiamonds, forming complexes that are capable of dispersing these drugs in water for sustained periods of time while also maintaining their functionality (confirmed via MTT and DNA fragmentation assays), whereas without the nanodiamonds, the drugs immediately precipitated and were rendered non-functional." | |
| Ho, an Assistant Professor in the Departments of Biomedical and Mechanical Engineering at the Robert R. McCormick School of Engineering and Applied Science at Northwestern University, has worked on this research with collaborators from Northwestern's Department of Mechanical Engineering, the Atomic and Nanoscale Characterization Experimental Center, and the Robert H. Lurie Comprehensive Cancer Center, as well as Shinshu University's NanoCarbon Research Institute. The team has reported their findings in the June 17, 2009 online edition of ACS Nano (Nanodiamond-Mediated Delivery of Water-Insoluble Therapeutics). | |
| We have already reported on Ho's previous work with nanodiamonds, where he has demonstrated a nanodiamond-embedded device that could be used to deliver chemotherapy drugs locally to sites where cancerous tumors have been surgically removed ("Nanotechnology cancer treatment with diamonds"). | |
| In this recent work, the team introduced the application of nanodiamonds towards the delivery of 4-Hydroxytamoxifen, an emerging breast cancer therapeutic typically soluble in solvents like ethanol, and Purvalanol A, a promising compound for liver cancer that is being challenged by the fact that is soluble in DMSO, a solvent that can preclude the clinical impact of the drug. | |
 |
|
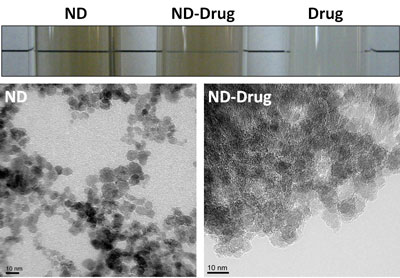
| Nanodiamonds were shown to enhance the ability to disperse Purvalanol A and 4-OHT in water. Scale bars represent 10 nm. (Image: Dr. Ho, Northwestern University) | |
| The researchers addressed several key challenges by introducing an approach that harnesses the biocompatibility of nanocarbon to package, release and main function of several drugs that are typically insoluble in water. This insolubility significantly challenges or potentially complicates their application in intravenous clinical settings because they often need to be injected into the bloodstream. In some extreme cases, the use of harmful solvents (e.g. DMSO) to dissolve the drugs and to accomplish the injections can immediately preclude their approval for human use, limiting the studies of their efficacy to animal models. | |
| Ho's team wanted to harness the integrative benefits of the nanodiamonds – e.g. biocompatibility, scalable processing parameters, dispersibility in water – to enable and accelerate the progression of multiple promising compounds currently impacted by their inability to disperse and remain functional in water, towards clinical applicability by dispersing them in water in a rapid fashion while preserving potent cancer-killing efficacy. | |
| Examining the nanodiamonds that they synthesized – using Fourier transform infrared spectroscopy (FTIR) – the scientists confirmed the presence of carboxyl groups on the surface, which were deposited as a result of acid treatment during the purification process to remove contaminants. | |
| "We hypothesized that the utility of the carboxyl groups contributes to the ability to interface the nanodiamonds with drug molecules through physisorption or electrostatic interactions such that the drug could eventually be released upon external stimuli," explains Ho. "In this study, we confirmed this hypothesis via a multitude of drug-nanodiamond imaging and characterization experiments and UV-vis analysis of drug-nanodiamond interfacing, in addition to functionality assays." | |
| The inability to solubilize promising cancer-fighting compounds in water can preclude their wide application in clinical use, drastically reducing the range of therapeutics that are available to enhance doctors' cancer-killing arsenal. This new work now shows a route towards the scalable delivery of water-insoluble drugs using the versatile nanodiamond platform using water-insoluble breast cancer and liver cancer-killing compounds as model therapeutics where rapid dispersibility in water and subsequent potent drug activity were demonstrated. | |
| The nanodiamond research field is moving towards the validation of clinical/translational efficacy. These experimental approaches are further being coupled with simulation/modeling efforts to better understand nanodiamond surface properties, material structure, as well as the density and spatial distribution of functional groups. These approaches will ultimately enable the optimization of drug loading onto the nanodiamonds, a better understanding and control over drug release, as well as the intelligent design of multi-drug, and stimuli-dependent release from the nanodiamond clusters. | |
| Ho notes that these significant advances will catalyze the progression of this field from a fundamental/benchtop domain towards pre-clinical/clinical efficacy and will be realized upon the integration of several disciplines, including the aforementioned modeling/simulation field, as well as bioengineering, materials science, chemistry, molecular biology, cancer biology, etc. "This integrative effort generates cross-disciplinary challenges, but also represents a major opportunity to impact the drug delivery and nanomedicine domains, among others." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
