| Sep 10, 2019 | |
Nanotechnology could be good for your heart |
|
| (Nanowerk Spotlight) Cardiovascular diseases (CVDs) are the number 1 cause of death globally. According to the World Health Organization (WHO) more people die annually from CVDs than from any other cause – an estimated 17.9 million people died from CVDs in 2016, representing 31% of all global deaths. Of these deaths, 85% are due to heart attack and stroke. | |
| "Among the various therapeutic advances, regenerative medicine and nanotechnologies have demonstrated a considerable capacity to salvage or regenerate damaged heart tissue in animal models," Morteza Mahmoudi, Assistant Professor, Precision Health Program at Michigan State University, tells Nanowerk. "The superior characteristics of nanobiomaterials have shown great promise in developing engineered cardiovascular constructs for a variety of tissue engineering applications. To develop efficient nanotechnology-based regenerative medicine platforms in humans, clinicians and engineers must achieve a greater common understanding of the problems, challenges, and opportunities in both fields." | |
| Mahmoudi is senior author of a recent review article that provides a comprehensive overview of the critical features of ischemic cardiovascular diseases (i.e. a restriction in blood supply to tissues), and emerging nanomedicine trends in the fields of cardiac nanotechnology, from the perspectives of both clinicians and bioengineers (Chemical Reviews, "Nanoscale Technologies for Prevention and Treatment of Heart Failure: Challenges and Opportunities"). | |
| This review discusses the potential application of nanotechnologies for addressing the challenges and limitations associated with the clinical application of current cardiac diagnostic and therapeutic approaches. | |
| "The development of highly effective cardiac regenerative therapies requires connecting and coordinating multiple fields, including cardiology, cellular and molecular biology, biochemistry and chemistry, and mechanical and materials sciences, among others," Mahmoudi points out. "In this paper, we intended to bridge the knowledge gap between cardiologists and regenerative nanomedicine experts. Establishing this multidisciplinary knowledge base may help pave the way for developing novel, safer, and more effective approaches that will enable the medical community to reduce morbidity and mortality in heart failure patients." | |
| The international team of authors list the main clinical challenges, which nanotechnologies may help to overcome in the field of heart failure and discusses relevant nanotech-based approaches (quoted verbatim from the article): | |
Robust Identification of Heart Failure Markers in the Blood |
|
| Our blood plasma contains over 10 000 proteins but 99% of the protein mass in the plasma proteome is dominated by a few tens of proteins. This means that robust identification of the disease-specific proteins/biomarkers that have a very low or rare abundance in plasma is challenging with the current proteomics approaches. Therefore, one of the crucial clinical diagnostic challenge is to detect heart failure biomarkers without false-negative and/or false-positive errors. | |
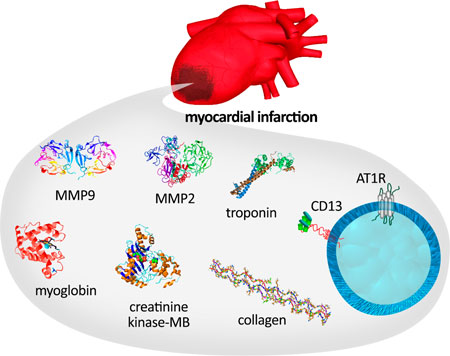 |
|
| Schematic representation of myocardial infarction-specific biomarkers. (Reprinted with permission from American Chemical Society) | |
Predicting Long-term Effects of Cardiac Injuries |
|
| Identification and discrimination of the level of cardiac injury and its long-term effects on cardiac function are of great clinical interest. This is because, in some cases, myocardial infarction only causes subtle injuries with initially negligible signs of adverse effects on heart function. In a fraction of these patients, in contrast to clinical expectations, substantial reductions in heart function are observed long-term (e.g., as early as a couple of months) after initial treatment. Although identification of the patients at risk of further cardiac damage in longer time is of clinical interest, there is currently no effective diagnostic approach for robust identification of these subpopulations of patients. | |
Delivering Therapeutic Molecules and/or Cells into the Damaged Part of Myocardium |
|
| Therapeutic molecules and/or cells must be delivered to the 'stunned' myocardium or the transient postischemic dysfunctional part of the heart tissue. The damaged cardiac cells in the stunned area have a unique capacity to retrieve their functions during the course of heart failure. Therefore, targeted delivery of therapeutic molecules and/or cells enables clinicians to minimize the scar tissue and maximize heart functions. However, current clinical strategies provide only limited success in targeted delivery of the therapeutic biosystems. | |
Low Therapeutic Cell Retention and Engraftment in Myocardium |
|
| The cell therapy approach has demonstrated great potential in retrieving cardiac tissue during heart failure. Theoretically, the therapeutic cells can integrate into the damaged cardiac cells and release their therapeutic paracrine factors to regenerate and heal the stunned part of myocardium. However, preclinical and clinical trials of cell therapy approaches thus far have found low retention and engraftment to the host cardiac tissue. | |
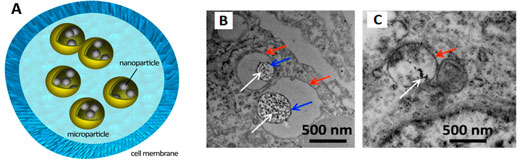 |
|
| (A) Schematic showing high-density and safe loading of iron oxide nanoparticles in therapeutic cells using biodegradable poly(lactide-coglycolide) microparticles. Transmission electron microscopy images of the labeled cells with (B) magnetic nanoparticles embedded in microcapsules (PLGA-MPs) and (C) magnetic nanoparticles alone (IO-NPs) are shown. White, blue, and red arrows show the locations of IO-NPs, PLGA-MPs, and membrane of intracellular compartment, respectively. (Reprinted with permission from American Chemical Society) (click on image to enlarge) | |
Patient-Specific Mature and Functional Cardiomyocytes |
|
| One of the main reasons for the low retention and engraftment of therapeutic cells is the role of the immune system in cell rejection. Development of patient specific cardiac cells is recognized as a useful strategy to overcome this problem. The patient-specific cardiac cells are mainly produced by differentiation of human pluripotent stem cells (hiPSCs). However, this process is plagued by the low maturity of the produced cells, which not only reduces their therapeutic efficacy and integration into the host tissue but also creates some serious side effects such as arrythmia. | |
Robust Monitoring of Therapeutic Cells in Vivo |
|
| Clinical monitoring of the therapeutic cells is very important to probe the fate of the cells. The sensitivity and specificity of current clinical strategies are not good enough to monitor therapeutic cells over the course of treatment. | |
Reperfusion Injury |
|
| Reperfusion injury is typified by vascular, myocardial, or electrophysiological dysfunction brought about by the return of bloodstream to ischemic tissue. When the stream of blood into cardiac myocytes is interrupted through the blocking of a coronary artery, a sequence of actions results in cellular injury and death. In the context of severe myocardial infarction, a report posited that reperfusion injury is responsible for up to 50% of the final myocardial damage. | |
| As the authors point out, although the field of cardiac nanotechnology is developing exponentially, and despite intriguing reports of both in vitro and in vivo studies (using a wide range of small and large animals), their successful clinical translation remains elusive. | |
| "The field of nanomedicine has largely overlooked factors that are present in both the in vitro and in vivo microenvironment without fully understanding the nanobio interface, resulting in reduced precision of estimation of the nanoparticles? fate and safety in human subjects," says Mahmoudi. "We and others have made extensive efforts to correct well-intentioned misinterpretations in the current literature, mainly through identifying and characterizing previously overlooked or unknown factors at the nanobio interface." | |
| The team concludes that building strong bridges between clinicians, bioengineers and nanotechnology experts is essential for the field to direct the research strategies with careful plans, rather than through scattered reports, that can address the main unmet clinical needs. | |
| As described in this review, many of the potential applications of nanotechnologies in heart failure capable of saving many lives remain poorly understood. This is caused by the absence of efficient communications among experts in different fields. | |
| The authors emphasize that effective communications and collaborations by scientists working in cardiac nanotechnology are imperative for achieving more accurate and precise prediction of the biological fate of nanomaterials, their safety, and therapeutic efficacy, all of which are instrumental in the ultimate goal of achieving successful clinical translation of nanotechnologies. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
