| Nov 17, 2020 | |
MBenes - boron-analogues of MXenes - enable efficient electrochemical synthesis of ammonia |
|
| (Nanowerk Spotlight) Ammonia (NH3) is widely used in industrial applications as a gas for refrigeration facilities, water purification, and in the manufacture of plastics, textiles, pesticides, and dyes as well as a building block for other chemicals. Notwithstanding all these other uses, about 90% of ammonia produced worldwide is used in fertilizers to help sustain food production for a growing global population. | |
| In biological systems, nitrogenases can efficiently convert N2 into bioavailable NH3 in an ATP-driven process called N2 fixation. The industrial production of NH3, however, relies on the energy-intensive Haber–Bosch process (N2 + 3H2 → 2NH3) over heterogeneous Fe/Ru-based catalysts or Co–Mo–N alloys – a process that causes significant environmental pollution. | |
| Researchers therefore have been exploring more efficient catalysts and alternative technologies. One of them is ammonia production through the electrochemical nitrogen reduction reaction (eNRR), which could provide a new way to substitute the Haber–Bosch process because it can be performed under ambient conditions and use renewable energy from wind and solar power. | |
| However, the electrocatalysts currently used in this reaction suffer from both high overpotential and poor catalytic selectivity due to the difficulty in activating the chemically inert triple bonds of N2 molecules and effectively suppressing competitive hydrogen evolution reaction (HER). | |
| 2D materials possess ultrahigh specific surface area and surface-to-volume atomic ratio, holding great promise for electrochemical reactions. Numerous 2D materials have already been developed as catalysts for HER, oxygen reduction/evolution reaction (ORR/OER), and CO2 reduction. | |
| However, only a small set of 2D electrocatalysts have so far been reported as suitable for NRR. In particular, the basal plane of most available 2D electrocatalysts is chemically inert for the N2 activation, making them inefficient towards NRR in their pristine form. Researchers found that the origin of activity and selectivity is primarily associated with their exposed edge sites or defective sites. | |
| Several strategies – such as doping, interfacial engineering, and defect modifications – have been employed to improve the specific activity of 2D materials. These techniques typically require strict optimization of the conditions to produce suitable doping or defects (e.g., the solvents, dopants, as well as doping concentrations), and the resulting catalysts suffer from limited active sites. | |
| "Although the past few years have witnessed the meteoric rise of yield rate as well as the Faradaic efficiency of this electrochemical process, the high catalytic reactivity of catalysts is limited to their edge or defect sites, while the majority of the surface is chemically inert," Prof.Zhongfang Chen from the University of Puerto Rico tells Nanowerk. "To achieve efficient ammonia synthesis via eNRR, a qualified catalyst should have both high specific activity and a large reaction area. However, integration of these two merits into one single material remains a big challenge due to the difficulty in balancing multiple reaction intermediates." | |
| In new work reported in Advanced Functional Materials ("Establishing a Theoretical Landscape for Identifying Basal Plane Active 2D Metal Borides (MBenes) toward Nitrogen Electroreduction"), a research team led by Prof. Shiping Huang (State Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing, China), Prof. Zhongfang Chen and Prof. Shengli Zhang (MIIT Key Laboratory of Advanced Display Materials and Devices, Ministry of Industry and Information Technology, Institute of Optoelectronics & Nanomaterials, Nanjing University of Science and Technology, Nanjing, China) proposed that a class of 2D transition metal borides termed MBenes – the boron-analogues of MXenes – could address this challenge and simultaneously achieve high activity and large reaction region toward eNRR. | |
| Using extensive density functional theory computations and taking 16 MBenes as representatives (see Figure 1), the team established a theoretical landscape of MBenes as eNRR catalysts by considering four key aspects: stability, activity, selectivity, with special attention to their surface phase diagrams under aqueous conditions. | |
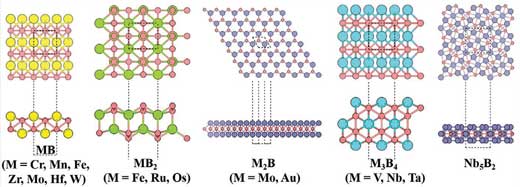 |
|
| Figure 1. Structural prototypes of MBenes. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| "We demonstrated that MBenes can effectively promote the adsorption and activation of N2 molecules, while suppressing the competitive hydrogen evolution reaction," says Xiangyu Guo, the first author of the paper. "Encouragingly, the notorious surface oxidation/degradation problem, which limits the catalytic performance of MXenes for NRR, can be significantly resolved on the surfaces of seven MBenes (CrB, MoB, WB, Mo2B, V3B4, CrMnB2 and CrFeB2), offering these catalysts intrinsic activity, selectivity, and a large reaction region toward the NH3 synthesis. In particular, the CrMnB2 MBene reached a record level of theoretical activity with a limiting potential of -0.22 V." | |
| Professor Chen adds: "Because the adsorption free energies of water molecules on MBenes are relatively small, well below 0.75 eV, they are typically considered as accessible for reactions occurring at room temperature (see Figure 2b). Once the H2O adsorbates are formed, the diffusion and desorption of H2O onto the MBene surfaces can then readily occur. In contrast, the desorption of H2O onto MXenes could be significantly prohibited due to the strong adsorption (>0.75 eV)." | |
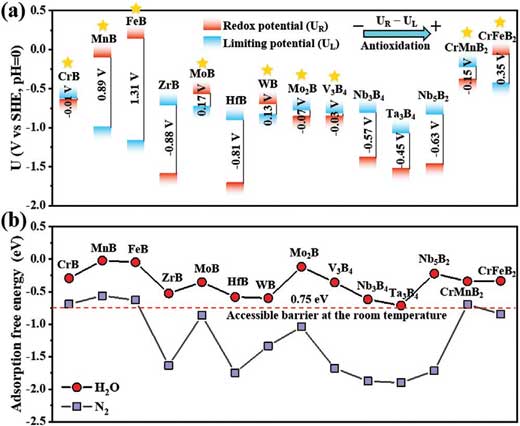 |
|
| Figure 2. a) Energy levels of theoretical limiting potential (UL) and redox potential (UR) of 14 MBenes, which satisfy the N2 adsorption criterion. The calculated values of UR - UL are also shown. b) Computed adsorption free energies of H2O and N2 on 14 MBene surfaces. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| "Since the redox potential, which is defined as the electrode potential needed to remove the surface O*/OH* species, is close to the theoretical limiting potential, the O*/OH* species may be easily reduced without applying external electrode potential," Guo points out. | |
| In addition, on the basis of the catalytic selectivity analysis, the team points out that these MBenes, including CrB, MoB, WB, Mo2B, V3B4, CrMnB2, and CrFeB2, exhibit the ability to suppress the competitive HER, and thus could achieve superior catalytic performance on their basal planes without introducing any dopants or defects. | |
| "Our work not only identifies efficient NRR electrocatalysts, but also provides a general design principle for further exploration of an even broader composition space of MBenes and other 2D NRR electrocatalysts, thus paving the way for advancing sustainable NH3 production," Chen concludes. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
