| Oct 06, 2023 | |
Unlocking nature's secrets yields powerful ionic generator |
|
| (Nanowerk Spotlight) Biological potassium channels are marvels of selective permeability, allowing rapid transport of potassium ions (ionic radius of 1.3 Å) while blocking the smaller sodium ions (1.0 Å). This ion selectivity and high conduction underpins nerve signaling and many other biological processes. Scientists have long sought to mimic these capabilities in artificial systems but have fallen far short of matching the exquisite ion discrimination of natural protein channels. | |
| Now, researchers in China have computationally designed artificial ion channels that approach the selectivity of their biological counterparts. As reported in a recent paper in National Science Review ("Designing artificial ion channels with strict K+/Na+ selectivity toward the-next-generation electric-eel-mimetic ionic power generation"), the team created nanopores in layered graphene sheets and decorated their edges with a ring of carbonyl groups. By computationally simulating ion transport through these pores, they demonstrated potassium ion conduction rates rivaling natural channels, while almost completely blocking sodium ions. | |
 |
|
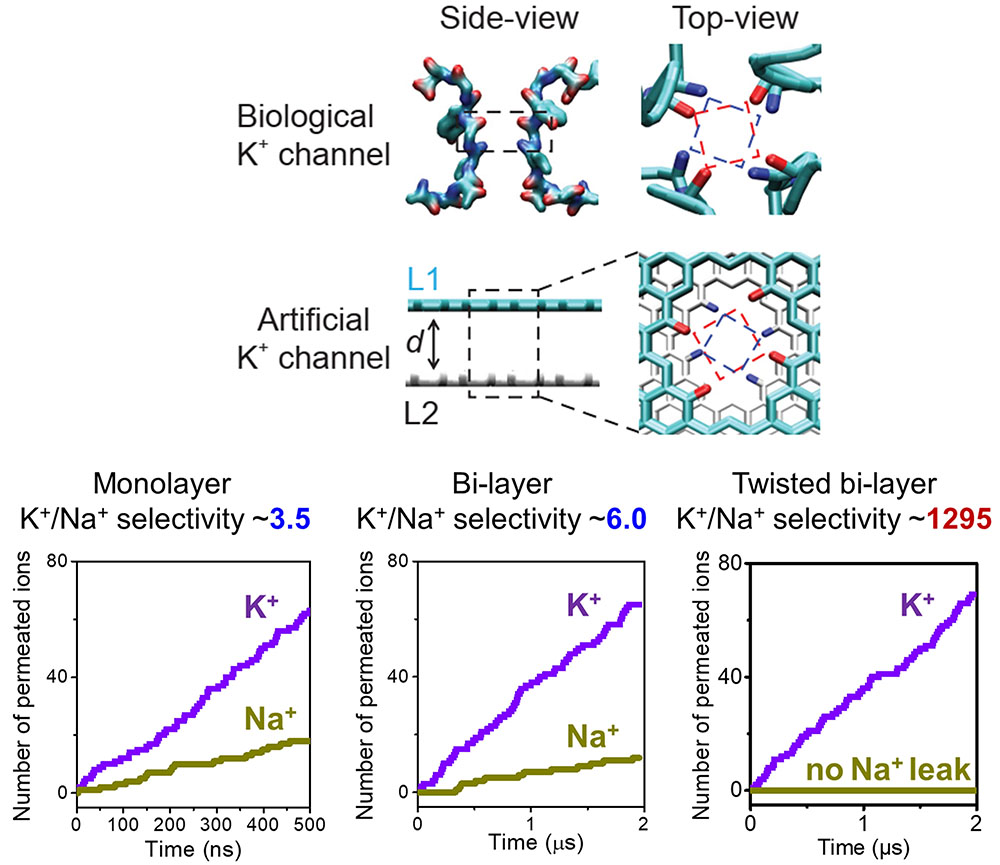
| Inspired by the rotary distribution of carbonyl rings in the selectivity filter of biological KcsA channels, a bi-layer graphene nanopore model with twisted carbonyl functionalization is established as an artificial potassium channel, and enables strict K+ selectivity. (Reprinted from National Science Review 2023, DOl: 10.1093/sr/nwad260 under CC BY 4.0) | |
| This extreme selectivity arises from two key design features. First, the nanopore is made of two graphene layers, which provides a confined space for ion and water coordination. Second, the carbonyl groups on each layer are twisted relative to each other, mimicking the arrangement found in potassium channels. | |
| "The long-overlooked structural feature of the rotary carbonyl rings underpins the ultra-high K+/Na+ selectivity, but has never been utilized in constructing artificial potassium channels," Wei Guo, professor at the Research Institute for Frontier Science, Beihang University, tells Nanowerk. | |
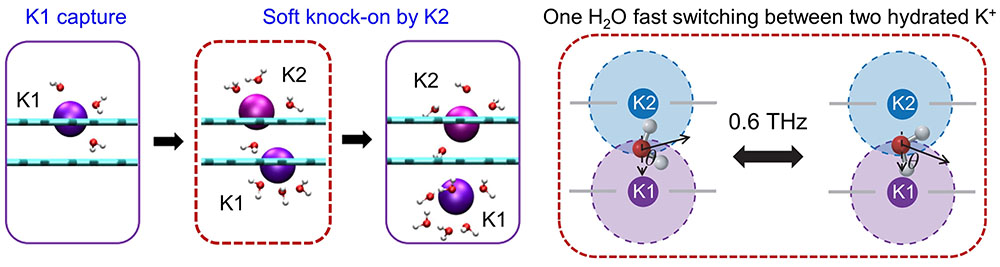
| Analysis of the simulated ion trajectories shows potassium ions moving through the bilayer pore two at a time. The carbonyl groups stabilize the ions in the confined space, while water molecules bridge them across the layers. This dual ion knock-on mechanism allows rapid potassium transport. | |
| In contrast, sodium ions tend to get stuck when they jump from the first layer to the second, blocked by their poor fit within the carbonyl rings. The simulation found a potassium-to-sodium selectivity of nearly 1300-to-1, compared to 40-to-1 or less for previous artificial designs. | |
 |
|
| The permeation of K+ follows a dual-ion transport mechanism, which is previously found only in biological ion channels. An intermediate water molecule rapidly switches between two hydrated K+ at a frequency of 0.6 THz, and connects the like-charged potassium ions together, termed K-H2O-K triplet. (Reprinted from National Science Review 2023, DOl: 10.1093/sr/nwad260 under CC BY 4.0) | |
| Beyond gaining insight into the workings of biological systems, highly selective artificial ion channels enable new applications not found in nature. As a proof of concept, the researchers propose using these graphene nanopores to harvest energy from electrolyte solutions of equal concentration. | |
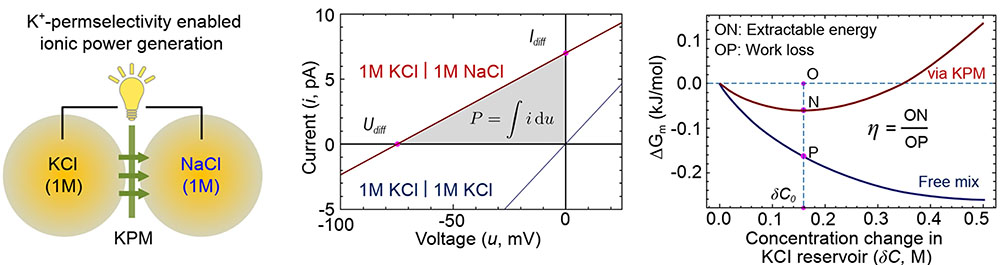
| Dubbed potassium-permselectivity enabled osmotic power generation (PoPee-OPG), the device would use membranes with Ångstrom-scale potassium-selective pores separating potassium chloride and sodium chloride solutions. Only potassium ions could cross the membrane, generating electrical current and power. | |
| Notably, PoPee-OPG could operate using solutions of equal total ionic strength. This contrasts the diluted solutions used in existing osmotic power designs, which limit their power density. The projected power density exceeds 1200 W/m2 at less than 1% nanopore coverage of the membrane. | |
 |
|
| Ionic power generation by mixing KCl and NaCl solutions of equal concentration. Only potassium ions can penetrate the K+-permselective membrane (KPM), thus, net diffusive ionic current from the KCl to the NaCl reservoir can be harvested as electric energy. Free energy change via a KPM or via a non-selective membrane (free mix) can be calculated for the energy conversion efficiency. (Reprinted from National Science Review 2023, DOl: 10.1093/sr/nwad260 under CC BY 4.0) | |
| "In our opinion, PoPee-OPG better mimics the way of energy conversion by the electrocyte cell of electric eels, and offers major advantages over existing salinity gradient power generation (SGPG)," says Guo. | |
| By eliminating the need for a diluted solution, the new approach greatly reduces internal resistance and enables high output power, much like the electric organs that give electric eels their shock. | |
| While still theoretical at this stage, the principles demonstrated could guide fabrication of highly selective membranes from stacked graphene-based materials. Ionic power generation is just one potential application; water purification, chemical separation, and improved batteries are others. | |
| "The unique pore structure proposed in this work provides valuable insights for synthesizing, for example, bi-layer covalent-organic framework membranes with precisely designed ion binding sites," Guo concludes. | |
| Overall, the biomimetic design principles yield ion channels with unprecedented selectivity and speed compared to artificial systems. They provide a blueprint for versatile applications and bringing the exceptional capabilities of natural proteins into human-engineered devices. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
