| Posted: Sep 20, 2016 | |
A novel approach for nanotoxicity evaluation in human cells using SERS |
|
| (Nanowerk Spotlight) Toxicity of nanomaterials is an emerging issue for public health and environmental safety. However, currently available toxicity screening methods are not fully compatible with nanotoxicity studies. | |
| Due to the unique physicochemical characteristics of nanomaterials, such as altered light absorption or scattering properties, the conventional cytotoxicity assays have been shown to create complications in nanotoxicity evaluation. Therefore, alternative methods that can reduce or replace the complications in nanotoxicity determination are highly desired. | |
| In a new study, published in the September 9, 2016 online edition of Analytical Chemistry ("Surface-Enhanced Raman Scattering to evaluate nanomaterial cytotoxicity on living cells"), researchers used surface-enhanced Raman scattering (SERS) to evaluate the cytotoxicity of nanomaterials. They show that SERS can be used as an alternative nanotoxicity evaluation method especially for the nanomaterials that have been shown to create complications in conventional cytotoxicity assays. | |
| In this vibrational spectroscopy technique, noble metals, such as gold, silver or copper, with rough surfaces are often used to enhance the Raman scattering effect up to ∼1014 times. SERS is a label-free technique, thus it can bypass the assay-related complications. | |
 |
|
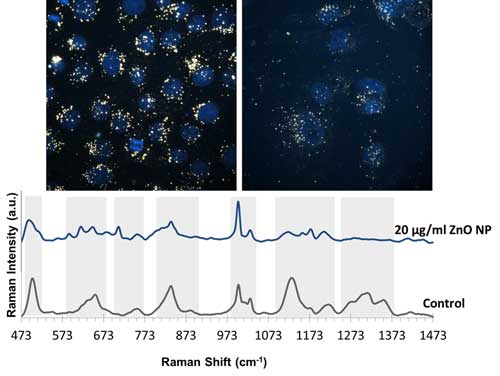
| Above images are enhanced-dark field images of A549 cells only incubated with gold nanoparticles and gold nanoparticles together with 20 µg/ml ZnO nanoparticles. Nuclei of the cells were stained with DAPI and are shown as blue in the image. Below SERS spectra belongs to the A549 cells only incubated with gold nanoparticles and gold nanoparticles together with 20 µg/ml ZnO nanoparticles (Image courtesy of the researchers)(click on image to enlarge) | |
| "We believe SERS can provide a deeper understanding of biomolecular changes in cells upon NM exposure," Mustafa Culha, a professor in the Genetics and Bioengineering Department of Yeditepe University in Turkey, tells Nanowerk. "It also provides a broader view on the cellular effects unlike conventional cytotoxicity studies where only one aspect of toxicity can be observed with one assay type. It is possible to gather information about cell death pathways, cellular stress levels as well as the state of cellular compartments by using SERS." | |
| In their study, the team used spherical gold nanoparticles of 50 nm diameter size as the SERS substrate. They observed cell-type and dose-dependent biochemical changes in the SERS spectra of tested samples compared to non-treated control samples. | |
| "We observed most of the changes in the lipid and protein-related bands of the spectra, indicating protein denaturation, protein defragmentation, lipid peroxidation, endoplasmic reticulum mitochondria co-localization all of which can be related to cellular stress and cell death," explains Culha. | |
| He notes that, although titanium dioxide (TiO2) nanoparticles are shown as mildly toxic in many studies as well as the conventional tests that the team used to correlate their findings, they observed protein structure-related stress indications in the SERS spectra. | |
| The transmission electron microscopy (TEM) and dark-field microscopy images also revealed extreme amounts of TiO2 nanoparticle accumulation. | |
| All of these findings provide an insight to mechanisms of in vitro nanotoxicity. | |
| The researchers point out that the vast majority of nanotoxicity studies in the literature cover only one aspect of the overall picture. | |
| "More mechanistic and systematic studies are needed to more accurately monitor the nanomaterial cytotoxicity," says Culha. "Although our study presents a semi-quantitative approach, the dose-dependent spectral information efficiently reveals the stages of nanotoxic response." | |
| "The distribution and status of gold nanoparticles, which can be considered as nanosensors reporting of local molecular changes, are critical for interpretation of the SERS spectra, adds Rawil F. Fakhrullin, professor, Bionanotechnology Group, Institute of Fundamental Medicine and Biology, Kazan Federal University, whose group cooperated with Culha's team on this work. "Since the behaviors of gold nanoparticles in living cells can be affected in the presence of other nanomaterials, it is important to visulize both in living cells. An important feature of this study is the use of enhanced darkfield microscopy which allowed us to visualise intracellular distribution of nanomaterials in 'wet' samples." | |
| By doing this, more specific information can be obtained from more specific locations from the cells. | |
| The scientists now are working on the deeper understanding of the cellular changes upon nanomaterial exposure. Other types of nanomaterials are being tested on other cell lines than the ones used in this study to investigate whether the method can be applied broadly to other widely used nanomaterials. | |
| They are also planning to obtain organelle-specific biochemical changes upon nanomaterial exposure. | |
| Upon interaction with cell culture medium, both the nanomaterials to be tested for their toxicity and gold nanoparticles that are used as SERS substrates are covered with a layer that mostly consists of proteins and this layer is called as protein corona. The protein corona around nanomaterials was shown to evolve and respond to changes around it (see our previous Nanowerk Spotlight: "Proteins interact with 'ultrasmall' nanomaterials in unique ways"). | |
| Since the SERS effect is obtained from a very close vicinity of the SERS substrate, it is important to make sure that the gold nanoparticles are distributed evenly in the cells to some extent. Characterization of protein corona upon nanomaterial exposure is also another area of interest for the team's work going forward. | |
| "It would be exciting to apply the technique on more types of nanomaterials to challenge its power and test whether SERS might face any difficulties in nanotoxicity testing," concludes Culha. "One interesting material would be quantum dots which have fluorescence properties and might result in fluorescence background in the SERS spectra. However, it is still possible to reduce the dose or extend the nanomaterials exposure time to get rid of the fluorescence effect." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
