| Posted: Jul 09, 2014 | ||||||||
Nanomaterial safety: An international collaboration on in vitro testing strategies |
||||||||
| (Nanowerk Spotlight) As a society, we are increasingly reliant on high technology products that are made with rare and expensive materials. They require high energy and material inputs, many of which are potentially harmful to workers and end users and problematic to manage responsibly across the life-cycle. The current path of global consumerism is not sustainable. While there is no internationally agreed definition, nanomaterials are substances that fall in the size range of 1 to 100 nanometers (one billionth of one meter) in at least one dimension, and by virtue of the small size they may behave differently in the body or in the environment as compared to macro-scale counterparts of the same material. | ||||||||
| Engineered nanoscale materials (ENM or 'nanomaterials') offer the potential to create safer and more effective products through the use of smaller quantities of improved performance materials. Currently nanomaterials are used to improve the performance of life-saving drugs and medical technologies, to make renewable energy more efficient, to make value added products from industrial waste streams, to improve food, packaging, to lightweight materials used in transportation systems, and to improve many of the personal care products that we use every day. Nanomaterial manufacture and use is expected to increase over the coming years and despite the widespread use of nanomaterials in a variety of consumer products, we are only beginning to understand the impacts of these emerging materials on our health and the environment. To this end, the University of Alberta’s Ingenuity Lab is collaborating with the Society for Risk Analysis to evaluate the potential to use alternative test strategies (ATS) to improve our ability to assess nanomaterial toxicity and environmental impact. | ||||||||
|
Standard in vivo toxicology test methods that depend heavily on the use of animals have long been used to assess chemical safety. Existing and novel in vitro and in silico test methods provide important alternatives to in vivo animal testing for chemicals and potentially for ENM. Genotoxicity tests, for example, are used to assess the mutagenic potential of chemicals or nanomaterials in the replication of DNA in cells. Driven in part by increasing market and regulatory requirements for safer and more sustainable products, large international infrastructure has developed for creating, testing and validating in vitro test methods, and its use is expanding to chemical and nanomaterial assessment (NSF, 2007). The goals of reducing, refining and replacing animal testing (the commonly cited 'three Rs') – resonate with key and diverse stakeholders including animal rights groups, the bioethics community, the pharmaceutical industry, regulatory agencies and the broader public. Despite nearly a decade of effort in the conduct toxicology and exposure research to inform the assessment of health and environmental risks of nanomaterials, major gaps remain in the ability to understand and quantify risks. While there is now a large body of published data on carbon nanotubes and metal oxide nanoparticles, concern has been raised that speculation about nanomaterial risk has hardened into an assumption that there are 'as-yet-to-be-discovered risks' that we must identify and manage (Maynard, 2014) that demands extensive testing. To ensure the appropriate management of risk, there is a need to develop standardized, reliable approaches to evaluate and classify novel substances through screening techniques and the use of test methods that can reliably inform decision makers about important priorities for additional testing that may be required (Oberdorster et al, 2005; Nel et al, 2013). ATS encompasses a variety of approaches that could address this need. |
||||||||
|
Lori Sheremeta, the Assistant Director of Ingenuity Lab in Edmonton Alberta and past Chair of the Society for Risk Analysis (SRA) Emerging Nanoscale Materials Specialty Group (ENMSG), is collaborating with U.S.-based nanomaterials risk expert Jo Anne Shatkin (an SRA Councilor and co-founder of the SRA ENMSG), Environment Canada, Health Canada, the SRA ENMSG and others on a Pilot Project with the Organization for Economic Co-operation and Development (OECD) Working Party on Manufactured Nanomaterials (WPMN) to develop a report on the State of the Science for ATS for nanomaterials, catalogue of existing and emerging ATS methods in a database; and develop a case study to inform workshop deliberations and expert recommendations. ATS approaches are regarded by many to have the potential for rapid screening of large numbers and types of materials. They can include a breadth of techniques including high throughput screening methods (HTS), high content screening, computational approaches, toxicogenomics, cell-based methods, in vitro assays and non-mammalian whole animal models. The emergence of ATS raises questions about how the results of these methods may be used for assessing the potential risks of ENM. For instance, ATS could be used in combination in a multiple models approach to evaluate new ENM in a number of rapid assays and compare with well-studied substances using in vivo testing; thereby identifying ENM for additional testing in a more strategic fashion than is possible through conventional testing approaches. Predictive models are developed by categorizing toxicological data through a concept of relational characteristics, with representative substances in a category used to infer toxicological information about other substances that can be assigned to the same category. The development of predictive models, particularly computational models, requires large amounts of standardized high quality data. Critical to their acceptance, ATS must have advantages over the current or traditional methods, including reliability in producing consistent results, and 'predictiveness' in correctly identifying potential for biological effects that would occur in humans or other species of interest. |
||||||||
|
The use of models to assess the toxicity of substances has a long history that developed over time to inform understanding of chemical effects. Mammalian studies represent earlier models that informed toxicity testing. As the number of chemicals in commerce grew, alternative methods were sought that could inform decision making more quickly and with less expense. With the advent of cell culturing methods, new models emerged. Over time, these approaches have grown to inform both human and ecological toxicity testing. Predictive models have emerged from a better understanding of the relationships between chemical properties and behavior in the body or the environment. In vitro and predictive methods represent alternatives to the more traditional toxicology testing involving studies in mammalian organisms. These methods continue to grow in complexity, and are frequently applied as part of a testing strategy. There are many international efforts to develop, as well as to validate and standardize, these methods for chemicals, including organizations such as the US National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (ICCVAM), the European Union Reference Laboratory European Centre for the Validation of Alternative Methods (EURL ECVAM), the Japanese Center for the Validation of Alternative Methods (JacVAM), the Korean Centre for the Validation of Alternative Methods (KoCVAM) and the OECD. There is wide recognition that the diversity of NMs renders it impractical to use traditional animal testing to evaluate safety, hence there is significant interest in assessing the performance of both existing and emerging alternative testing strategies for NMs. Further, the EU directive REACH (Directive 2006/121/EC) requires replacing in vivo testing, and there is widespread popular agreement about the desire to limit animal testing. Finally, there is a need for more biologically informative toxicology methods (Hartung, 2010; Silbergeld et al, 2011; Landsiedel et al, 2009). The Society for Risk Analysis (SRA), through the Emerging Nanoscale Materials Specialty Group, is organizing a public workshop on September 15-16, 2014 in Washington, D.C. to investigate the use of ATS in risk analysis for nanoscale materials. The workshop will convene a diverse group of international experts and stakeholders to discuss how existing and novel in vitro assays may be applied in a 'multiple models' approach to inform the risk assessment of novel nanoscale materials in assessing hazard, potency and exposure potential. This effort builds on past SRA efforts and other recent expert meetings regarding the development and use of HTS by examining the availability and applicability of existing and novel ATS methods for a multiple models approach to toxicity, environmental and exposure analysis of nanomaterials in the risk analysis paradigm (Shatkin et al, 2010). The SRA Nano Risk Analysis II workshop seeks to elaborate shared strengths and gaps in support of a weight of evidence approach relying on ATS to inform context – specific decisions about risk from exposure to novel nanoscale materials. The goal is to inform the development of guidance for ATS use in overall approaches to those decisions. |
||||||||
| The main objectives of the workshop are to: | ||||||||
|
|
||||||||
| This workshop will facilitate collaboration and communication between experts working on ATS and those who will use the resulting data for risk analysis and risk management decisions. As a non-governmental organization, the Society for Risk Analysis is positioned to bring the diverse researchers, nanotechnology experts, users and concerned parties together to identify how to move forward on the critical need to conduct screening of novel and emerging nanoscale materials to overcome the current 'case-by-case' evaluation approach that has proven too intensive, contentious, and a barrier to commercialization of safer substances. The published research, education, and policy recommendations that result from this meeting, as well as the interpersonal networks established are expected to substantially influence the international nanotechnology research enterprise and emerging public policy for more sustainable management of NMs for the next decade. | ||||||||
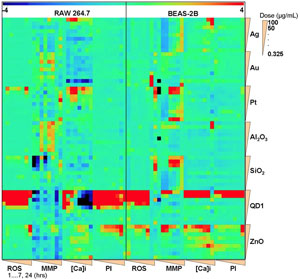
| In the United States, the U.S. ToxCast program has, as part of their 21st century toxicity screening program (NRC, 2007), tested 29 NMs with 62 in vitro test methods (Wang et al. 2013). Many researchers, including several from the University of Alberta, have proposed and developed ATS to include a variety of methods, some which are standardized for chemicals, and others which take advantage of developments including advanced biological mechanistic understanding, genomics, metabolomics, automation and informatics. However, these existing as well as emerging ATS have a short history with nanomaterials, and have not yet proven to be reliable for quantitative estimation of ENM risk. Still, several international efforts have developed ATS that have potential to be used for screening purposes, and to guide further testing priorities for regulatory decision making. The goal of the September workshop by the Society for Risk Analysis is to explore ways in which distinct ATS may be used for screening and prioritizing the need for more extensive testing of novel ENM. | ||||||||
|
||||||||
| References | ||||||||
| Hartung T, 2010. Food for thought on alternative methods for nanoparticle safety testing. Altex 27, 87-95. | ||||||||
| Landsiedel et al, 2010. Testing metal-oxide nanomaterials for human safety. Advanced Materials 22, 2601-2627. | ||||||||
| Maynard AD, 2014. A decade of uncertainty. Nature Nanotechnology 9, 159-160. | ||||||||
| Nel AE et al, 2013. A multi-stakeholder perspective on the use of alternative test strategies for nanomaterial safety assessment. ACS Nano 7, 6422-6433. | ||||||||
| NRC, 2007. Toxicity testing in the 21st century – a vision and a strategy. Washington D.C.: National Academies Press. | ||||||||
| Oberdorster et al, 2005. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives 113, 823-839. | ||||||||
| Sibergeld EK et al, 2011. Nanotoxicology: The end of the beginning – signs on the roadmap to a strategy for assuring the safe application and use of nanomaterials. Altex 28, 236-241. | ||||||||
| Shatkin JA et al, 2010. Nano risk analysis: advancing the science for nanomaterials risk management. Risk Analysis 30, 1680-1687. | ||||||||
| Wang et al. 2013. Ranking and profiling nanomaterial (NM) bioactivity by ToxCast high-throughput screening (HTS) (pdf). Society for Toxicology Meeting. | ||||||||
|
By Jo Anne Shatkin (President, Vireo Advisors, LLC, PO Box 51368, Boston, MA 02205 USA) and Lorraine Sheremeta (Assistant Director, Ingenuity Lab, University of Alberta, 1-070C, 11421 Saskatchewan Drive Edmonton, Alberta T6G 2M9)
|
||||||||
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
||||||||